Wintecare TECAR Therapy: What It Is, How It Works, and Who Benefits Most

Wintecare TECAR Therapy: What It Is, How It Works, and Who Benefits Most
When pain, tightness, or inflammation is slowing you down, one of the goals in a chiropractic setting is to help your body calm irritation and restore normal motion—as quickly and comfortably as possible. That’s why our office uses Wintecare TECAR (also called C.R.E.T.) therapy, a form of therapeutic radiofrequency/diathermy designed to support recovery in muscles, tendons, joints, and even deeper tissues. Wintecare+1
Below is an informative breakdown of what Wintecare is, what it may help with, and the types of patients who typically qualify.
What is Wintecare TECAR (C.R.E.T.) therapy?
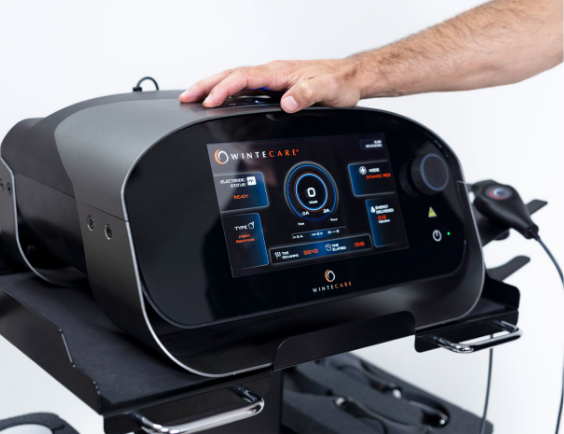
Wintecare is a company that develops physical therapy technologies, including TECAR/C.R.E.T. devices such as the T~PLUS, which uses an alternating current at a fixed frequency (commonly referenced as 0.448 MHz) delivered through capacitive and resistive electrodes with a return plate. Wintecare+1
In simple terms: Wintecare TECAR therapy delivers controlled radiofrequency energy into tissues. Depending on the settings and electrode type, the clinician can emphasize more superficial or deeper tissue effects. FDA Access Data+1
How it works (in plain English)
Most people understand “heat therapy,” but TECAR is often used because it can deliver energy deeper and more specifically than a basic heating pad—while still being comfortable.
Clinically, TECAR is used to support:
- Improved local circulation
- Reduction of muscle guarding/tension
- Easing pain and improving mobility
- Faster functional recovery when combined with movement and rehab
Many TECAR systems can also use pulsed modes, which can help manage heat build-up and make treatment more adaptable for more sensitive or acute presentations. Vitalis Physiotherapy
Benefits patients often notice
Every person responds differently, but patients commonly report benefits like:
1) Pain relief that helps you move again
When pain makes you stiff and guarded, even simple movement can feel “unsafe.” TECAR is often used to help decrease that protective tension so you can move more normally and tolerate rehab better.
2) Faster recovery for muscle and tendon issues
For strains, overuse injuries, and “tight and sore” muscles, TECAR is commonly used to help calm symptoms and improve tissue readiness—especially when paired with corrective exercise and hands-on care.
3) Support for joint aches and degenerative issues
Wintecare lists applications including degenerative joint conditions (like osteoarthritis) and general joint/musculoskeletal pain. Wintecare
4) Helpful for spine-related pain patterns
Wintecare also lists applications for low back, mid-back, and neck pain—with or without radiculopathies (radiating symptoms). Wintecare
5) Comfortable, relaxing sessions
Most people describe a gentle warming sensation and a “looser” feeling afterward—especially in chronically tight areas (hips, low back, shoulders, neck).
Who typically qualifies for Wintecare TECAR therapy?
We consider Wintecare TECAR when someone’s symptoms and exam suggest they’ll benefit from tissue calming + improved motion, such as:
Great-fit candidates often include:
- Athletes dealing with muscle tightness, overuse pain, or recovery needs (training, competitions, repetitive load)
- Desk workers with chronic neck/upper-back tightness and tension patterns
- Patients with low back pain or neck pain where muscle guarding and stiffness are a major contributor Wintecare
- People with joint aches (including degenerative joint pain) who do best with a combination of manual therapy + targeted movement Wintecare
- Anyone who needs a treatment that helps them tolerate rehab exercises better (because rehab is often the long-term win)
Who may NOT qualify (safety screening)
TECAR therapy is not appropriate for everyone. A clinical commentary and common clinical guidelines list key contraindications/precautions such as:
- Pregnancy (especially over abdominal/pelvic regions) PMC+1
- Pacemakers or implanted electronic devices (risk of interference) PMC+1
- Known/suspected malignancy in the treatment area PMC+1
- Active infection in the area PMC+1
- Thrombophlebitis / DVT concerns (circulatory changes may pose risk) PMC+1
This is why we screen your health history carefully before recommending it.
What a Wintecare session feels like
A typical session is comfortable and straightforward:
- We apply the appropriate electrode type (capacitive/resistive depending on the goal). FDA Access Data
- Most patients feel gentle warmth and a gradual softening of tight tissues.
- Treatment is often combined with other care (adjustments, soft tissue work, stretching, strengthening) to make the results more meaningful and longer lasting.
The “best results” approach: TECAR + movement
In our experience, TECAR is most valuable when it helps you do the next steps better—like:
- moving with less pain,
- restoring normal range of motion,
- progressing strengthening/stability work,
- returning to sport or daily life with fewer flare-ups.
Think of it as a tool that helps your body “turn down the noise,” so the real rehabilitation work can stick.
Curious if you’re a candidate?
If you’re dealing with pain, stiffness, inflammation, or an injury that isn’t improving the way you’d expect, we can evaluate whether Wintecare TECAR therapy is a fit—or whether a different approach would be safer and more effective for your specific case.
If you tell me which Wintecare model you have (e.g., T~PLUS or T~ADVANCED) and the conditions you most commonly treat (sports injuries, low back pain, neck pain, post-op rehab, etc.), I can tailor this blog post to match your exact services and add a strong call-to-action + FAQ section.
how many are in the US?
Thought for 40s
I couldn’t find any public, reliable number for how many Wintecare (e.g., T~PLUS / TECAR) units are installed in the United States—Wintecare doesn’t publish a U.S.-specific installation count on their site, and the FDA 510(k) clearance documents list the device and indications but don’t report how many are in use. FDA Access Data+2FDA Access Data+2
The closest official figure I found is Wintecare’s general statement that TECAR technology has “thousands of devices” installed worldwide, but it doesn’t break that down by country. Wintecare




